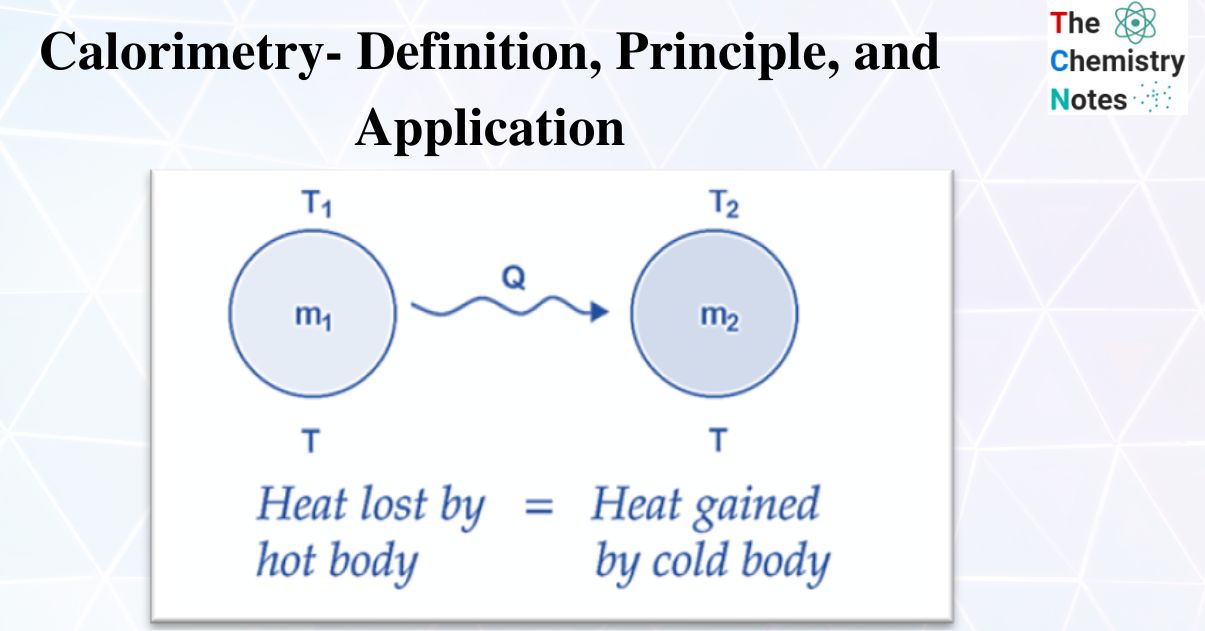
Calorimetry Data Definition . calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. apply the first law of thermodynamics to calorimetry compare heat flow from hot to cold objects in an ideal calorimeter. calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. To do so, the heat is exchanged with a calibrated object. To do so, the heat is. calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid.
from thechemistrynotes.com
For example, when an exothermic reaction. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid. To do so, the heat is exchanged with a calibrated object. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. apply the first law of thermodynamics to calorimetry compare heat flow from hot to cold objects in an ideal calorimeter. To do so, the heat is.
Calorimetry Definition, Principle, Types, Application, and Limitations
Calorimetry Data Definition For example, when an exothermic reaction. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. apply the first law of thermodynamics to calorimetry compare heat flow from hot to cold objects in an ideal calorimeter. To do so, the heat is exchanged with a calibrated object. calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid. To do so, the heat is. For example, when an exothermic reaction.
From exojtwjwx.blob.core.windows.net
Calorimetry Definition In Your Own Words at Earl Knight blog Calorimetry Data Definition To do so, the heat is exchanged with a calibrated object. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. To do so, the heat is. calorimetry is used to measure amounts of heat transferred to or from a substance. apply the first law of thermodynamics to calorimetry compare. Calorimetry Data Definition.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Data Definition calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry literally means. Calorimetry Data Definition.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Data Definition calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. To do so, the heat is.. Calorimetry Data Definition.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimetry Data Definition For example, when an exothermic reaction. calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid. apply the first law of thermodynamics to calorimetry compare heat flow from hot to cold objects in an ideal calorimeter. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry. Calorimetry Data Definition.
From thechemistrynotes.com
Calorimetry Definition, Principle, Types, Application, and Limitations Calorimetry Data Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is the. Calorimetry Data Definition.
From www.researchgate.net
Differential scanning calorimetry data types hydrated for 30 min and Calorimetry Data Definition calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is. For example, when an exothermic reaction. calorimetry is used to measure amounts of heat transferred to or from a substance.. Calorimetry Data Definition.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimetry Data Definition For example, when an exothermic reaction. calorimetry is used to measure amounts of heat transferred to or from a substance. apply the first law of thermodynamics to calorimetry compare heat flow from hot to cold objects in an ideal calorimeter. calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid. calorimetry. Calorimetry Data Definition.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimetry Data Definition calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid. calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring the amount. Calorimetry Data Definition.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Data Definition calorimetry is used to measure amounts of heat transferred to or from a substance. apply the first law of thermodynamics to calorimetry compare heat flow from hot to cold objects in an ideal calorimeter. To do so, the heat is. calorimetry is used to measure amounts of heat transferred to or from a substance. For example, when. Calorimetry Data Definition.
From study.com
Calorimetry Definition, Equation & Types Lesson Calorimetry Data Definition To do so, the heat is exchanged with a calibrated object. calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid. calorimetry is used to measure amounts of heat transferred to or from a substance. apply the first law of thermodynamics to calorimetry compare heat flow from hot to cold objects in. Calorimetry Data Definition.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Data Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To do so, the heat is. apply the first law of thermodynamics to calorimetry compare heat flow from hot to cold objects in an ideal calorimeter. calorimetry is used to measure amounts of heat transferred to or from. Calorimetry Data Definition.
From chem.libretexts.org
7.3 Heats of Reactions and Calorimetry Chemistry LibreTexts Calorimetry Data Definition apply the first law of thermodynamics to calorimetry compare heat flow from hot to cold objects in an ideal calorimeter. To do so, the heat is exchanged with a calibrated object. For example, when an exothermic reaction. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry literally means. Calorimetry Data Definition.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimetry Data Definition calorimetry is used to measure amounts of heat transferred to or from a substance. For example, when an exothermic reaction. apply the first law of thermodynamics to calorimetry compare heat flow from hot to cold objects in an ideal calorimeter. To do so, the heat is. calorimetry is used to measure amounts of heat transferred to or. Calorimetry Data Definition.
From thechemistrynotes.com
Calorimeter Definition, Types and Uses Calorimetry Data Definition calorimetry is used to measure amounts of heat transferred to or from a substance. apply the first law of thermodynamics to calorimetry compare heat flow from hot to cold objects in an ideal calorimeter. calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid. a calorimeter is a device used to. Calorimetry Data Definition.
From studylib.net
Calorimetry Calorimetry Data Definition For example, when an exothermic reaction. apply the first law of thermodynamics to calorimetry compare heat flow from hot to cold objects in an ideal calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To do so, the heat is exchanged with a calibrated object. calorimetry. Calorimetry Data Definition.
From www.studocu.com
Calorimetry CALORIMETRY Report sheet A. Determination of the Calorimetry Data Definition calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. To do so, the heat is. For example, when an exothermic reaction. To do so, the heat is exchanged with a calibrated object. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used. Calorimetry Data Definition.
From chem.libretexts.org
5.3 Calorimetry Chemistry LibreTexts Calorimetry Data Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is. Calorimetry Data Definition.
From www.studypool.com
SOLUTION Chemistry cit u calorimetry definition examples sample Calorimetry Data Definition apply the first law of thermodynamics to calorimetry compare heat flow from hot to cold objects in an ideal calorimeter. calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is. calorimetry. Calorimetry Data Definition.